Definitions and interpretations
Source: Canadian Legal Information Institute (CanLII). Controlled Drugs and Substances Act, S.C. 1996, c. 19, as amended
Source: L. A. King, Forensic Chemistry of Substance Misuse: A Guide to Drug Control, RSC Publishing, Cambridge, 2009
|
||||||||||||
| SHORT TITLE | ||||||||||||
| Short title | 1. This Act may be cited as the Controlled Drugs and Substances Act. | |||||||||||
| INTERPRETATION | ||||||||||||
| Definitions | 2. (1) In this Act, | |||||||||||
| “adjudicator” | “adjudicator” means a person appointed or employed under the Public Service Employment Act who performs the duties and functions of an adjudicator under this Act and the regulations; | |||||||||||
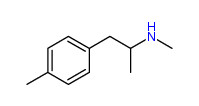
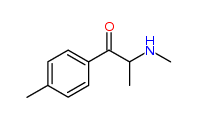
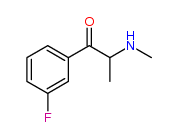
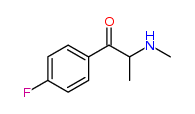
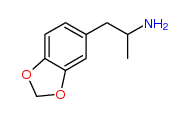
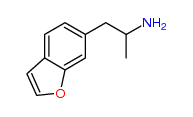
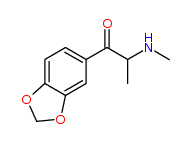
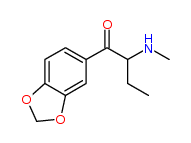
| “analogue” | “analogue” means a substance that, in relation to a controlled substance, has a substantially similar chemical structure;
The term “analogue” is employed in the following sections:
|
On 6 Apr 2010 I received an e-mail from Health Canada in response to my inquiry about Mephedrone, 3-Fluoromethcathione and 4-Fluoromethcathione:
On 13 Sept 2010 a reader kindly shared Health Canada’s opinion in answer to a query about 6-APB:
In January 2011 I learned that Health Canada considers these four substances controlled:
I have not seen any documents setting out their rationale, but
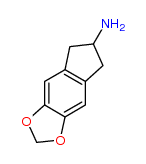
I imagine the first two substances are deemed analogues of N-Methyl-3,4-methylenedioxyamphetamine (N,α-Dimethyl-1,3-benzodioxole-5-ethanamine) (a.k.a. MDMA or Ecstasy), listed at Schedule III section 1 subsection (9).
It might be argued the third, MDAI, is an analogue of 3,4-Methylenedioxyamphetamine (α-Methyl-1,3-benzodioxole-5-ethanamine) listed at Schedule III section 1 subsection (5).
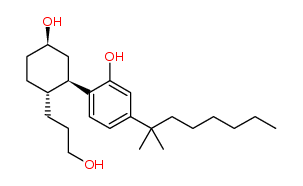
CP 55,940 is a synthetic cannabinoid (i.e. a substance that binds to cannabinoid receptors),
one of hundreds synthesized in recent decades. These substances, to one degree or another,
are structurally or pharmacologically related to the naturally occurring cannabinoids found in Cannabis such as Tetrahydrocannabinol, listed at Schedule II section 1 subsection (7).
The only possible basis for considering CP 55,940 controlled is found at Schedule II section 1 which reads “Cannabis its preparations, derivatives and similar synthetic preparations” (emphasis added). Since it is neither a preparation nor a derivative of Cannabis, Health Canada presumably feels that “similar synthetic preparations” includes CP 55,940.
These examples reveal the Act’s highly subjective definition of “analogue” to be a remarkably elastic and adaptable device.
Highly useful from a regulatory standpoint—dangerously arbitrary when embedded in criminal law.
Particularly when, as is Health Canada’s custom,
their “interpretations” are never made public.
When employed by a chemist, analogue is a handy term, used routinely and casually to highlight
a common structural motif across a group of substances. It signifies a relationship between given substances;
it does not define which substances are so related and which are not.
Ask a chemist to list, for instance, the analogues of penicillin; I’ll wager all you’ll get back is a bewildered silence.
The list of every substance they have considered and their conclusion as to whether it is controlled or not can be viewed here. I want to state clearly and pointedly: this is not law—it is Health Canada’s opinion and, as far as I know, untested in the courts. I’ve posted it because I believe it best that everyone have this information. Please do not ask me to interpret this data; please ask Health Canada instead.
Using the Access to Information Act (ATI) I’ve tried to shine a light inside Health Canada’s “Skull and Bones” crypto-culture of concealment. These may help illustrate how things work, or not, at Health Canada. I warn you though, public servants signal (I hesitate to say “communicate”) using an arcane, often impenetrable code. Remarkably, other public servants are paid to interpret this code, and have been so proactive and transparent as to make it available even to the public: Treasury Board Newspeak Dictionary.
|
||||||||||
| “analyst” | “analyst” means a person who is designated as an analyst under section 44; | |||||||||||
| “Attorney General” | “Attorney General” means
|
|||||||||||
| “controlled substance” | “controlled substance” means a substance included in Schedule I, II, III, IV or V; | |||||||||||
| “derivative” | “It is now usually accepted amongst forensic chemists that
compound A is a derivative of compound B only if B can be
converted to A in a single chemical reaction,
even if that is only achievable in a theoretical sense.”
“Derivative” is employed but not defined in the CDSA.
The definition above is from King, pp 20–21.
|
|||||||||||
| “designated substance offence” | “designated substance offence” means
|
|||||||||||
| “inspector” | “inspector” means a person who is designated as an inspector under section 30; | |||||||||||
| “isomer” |
The term “isomer,” not defined in the Act, is employed in the following sections:
A good analogy can be found in language. With words and letters standing for molecules and atoms, all of these are isomers: part, trap, rapt, tarp, and every other arrangement of those four letters whether found in a dictionary or not. In chemical terms, what the law actually says makes no sense whatsoever, when even a small molecule like amphetamine has hundreds of known isomers (i.e. ones synthesized and reported in the literature) and thousands more potential isomers. |
|||||||||||
| “judge” | “judge” means a judge as defined in section 552 of the Criminal Code or a judge of a superior court of criminal jurisdiction; | |||||||||||
| “justice” | “justice” has the same meaning as in section 2 of the Criminal Code; | |||||||||||
| “Minister” | “Minister” means the Minister of Health; | |||||||||||
| “offence-related property” | “offence-related property” means, with the exception of a controlled substance, any property, within or outside Canada,
|
|||||||||||
| “possession” | “possession” means possession within the meaning of subsection 4(3) of the Criminal Code; | |||||||||||
| “practitioner” | “practitioner” means a person who is registered and entitled under the laws of a province to practise in that province the profession of medicine, dentistry or veterinary medicine, and includes any other person or class of persons prescribed as a practitioner; | |||||||||||
| “precursor” | “precursor” means a substance included in Schedule VI; | |||||||||||
| “prescribed” | “prescribed” means prescribed by the regulations; | |||||||||||
| “produce” | “produce” means, in respect of a substance included in any of Schedule I to IV, to obtain the substance by any method or process including
|
|||||||||||
| “provide” | “provide” means to give, transfer or otherwise make available in any manner, whether directly or indirectly and whether or not for consideration; | |||||||||||
| “salt” |
“Salt” is liberally employed, but not defined, in the Act.
|
|||||||||||
| “sell” | “sell” includes offer for sale, expose for sale, have in possession for sale and distribute, whether or not the distribution is made for consideration; | |||||||||||
| “traffic” | “traffic” means, in respect of a substance included in any of Schedules I to IV,
|
|||||||||||
| Interpretation | 2. (2) For the purposes of this Act,
|
|||||||||||
| Interpretation | 2. (3) For the purposes of this Act, where a substance is expressly named in any of Schedule I to VI, it shall be deemed not to be included in any other of those Schedules. | |||||||||||
| Interpretation | 3. (1) Every power or duty imposed under this Act that may be exercised or performed in respect of an offence under this Act may be exercised or performed in respect of a conspiracy, or an attempt to commit, being an accessory after the fact in relation to, or any counselling in relation to, an offence under this Act. | |||||||||||
| Interpretation | 3. (2) For the purposes of sections 16 and 20, a reference to a person who is or was convicted of a designated substance offence includes a reference to an offender who is discharged under section 730 of the Criminal Code. | |||||||||||
| Interpretation | 5. (5) For the purposes of applying subsection (3) or (4) in respect of an offence under subsection (1), a reference to a substance included in Schedule I, II, III or IV includes a reference to any substance represented or held out to be a substance included in that Schedule. | |||||||||||
| Interpretation | 5. (6) For the purposes of subsection (4) and Schedule VII, the amount of the substance means the entire amount of any mixture or substance, or the whole of any plant, that contains a detectable amount of the substance. | |||||||||||
 · Isomer Design
· Isomer Design