Acetyldihydrocodeine
CA: CDSA Schedule I Section 1 Subsection 6 Acetyldihydrocodeine
UN: Narcotics Part 1 Section 2 Acetyldihydrocodeine and Part 2 Section 1 Acetyldihydrocodeine
US: CSA Schedule I Section c Subsection 2 Acetyldihydrocodeine
Amphetamine
CA: CDSA Schedule I Section 19 Subsection 1 Amphetamine
CA: [revoked] CDSA Schedule III Section 1 Subsection 1 Amphetamine
UN: Psychotropics Schedule II Amfetamine (Amphetamine)
US: CSA Schedule II Section d Subsection 1 Amphetamine
⇒
PubChem: 3007; ChemSpider: 13852819; Bluelight: Amphetamine; Drugs Forum: Amphetamine; Erowid: Amphetamine; Wikipedia: AmphetamineEffective 26 Jan 2009, S.I. 2008/3130 added:
Cannabinol
CA: CDSA Schedule II Section 1 Subsection 4 Cannabinol
See also MDA Part II Class B Section 1(a) Cannabinol
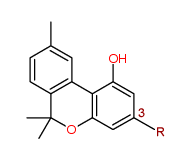
Cannabinol
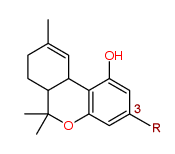
Tetrahydrocannabinol
where R = pentyl = C5H11
where R = pentyl = C5H11
Effective 26 Jan 2009, S.I. 2008/3130 added:
Cannabinol derivatives
[Cannabinol derivatives means the following substances, except where contained in cannabis or cannabis resin, namely tetrahydro derivatives of cannabinol and 3-alkyl homologues of cannabinol or of its tetrahydro derivatives.]
CA: CDSA Schedule II Section 1 Subsection 4 Cannabinol
See also MDA Part II Class B Section 1(a) Cannabinol
CA: CDSA Schedule II Section 1 Subsection 7 Tetrahydrocannabinol
US: CSA Schedule I Section d Subsection 31 Δ1 cis or trans Tetrahydrocannabinol
⇒
PubChem: 16078; ChemSpider: 4479482; Bluelight: Tetrahydrocannabinol; Erowid: Cannabis; Wikipedia: TetrahydrocannabinolEffective 26 Jan 2009, S.I. 2008/3130 added:
Effective 29 Jan 2004, S.I. 2003/3201 revoked:
Cannabis and Cannabis resin
CA: CDSA Schedule II Section 1 Subsection 2 Cannabis, Schedule VII Section 2 Cannabis and Schedule VIII Section 2 Cannabis
US: CSA Schedule I Section d Subsection 23 Marihuana
CA: CDSA Schedule II Section 1 Subsection 1 Cannabis resin, Schedule VII Section 1 Cannabis resin and Schedule VIII Section 1 Cannabis resin
UN: Narcotics Part 1 Section 1 Cannabis resin and Section 3 Cannabis resin
Codeine
CA: CDSA Schedule I Section 1 Subsection 2 Codeine
Effective 1 Apr 1986, S.I. 1985/1995 revoked:
Dexamphetamine
UK: Currently at MDA Part II Class B Section 1(a) Amphetamine
Dihydrocodeine
CA: CDSA Schedule I Section 1 Subsection 11 Dihydrocodeine
UN: Narcotics Part 1 Section 2 Dihydrocodeine and Part 2 Section 1 Dihydrocodeine
US: CSA Schedule II Section c Subsection 7 Dihydrocodeine, Schedule III Section e Subsection 1 Item iii Dihydrocodeine and Schedule V Section c Subsection 2 Dihydrocodeine
Ethylmorphine (3-Ethylmorphine)
CA: CDSA Schedule I Section 1 Subsection 13 Ethylmorphine
UN: Narcotics Part 1 Section 2 Ethylmorphine and Part 2 Section 1 Ethylmorphine
US: CSA Schedule II Section b Subsection 1 Item iii Ethylmorphine, Schedule III Section e Subsection 1 Item iv Ethylmorphine and Schedule V Section c Subsection 3 Ethylmorphine
Effective 1 Apr 1986, S.I. 1985/1995 added:
Glutethimide
CA: CDSA Schedule IV Section 11 Glutethimide
UN: Psychotropics Schedule III Glutethimide
US: CSA Schedule II Section e Subsection 2 Glutethimide
Effective 10 Jun 2014, S.I. 2014/1106 added:
On 5 Mar 2014, S.I. 2014/1106* proposed to add:
Ketamine
Effective 1 Apr 1986, S.I. 1985/1995 added:
Lefetamine
CA: CDSA Schedule III Section 29 Lefetamine
UN: Psychotropics Schedule IV Lefetamine (SPA)
US: CSA Schedule IV Section f Subsection 12 SPA
⇒
PubChem: 22779; ChemSpider: 21356; Bluelight: Lefetamine; Drugs Forum: Lefetamine; Wikipedia: LefetamineEffective 10 Jun 2014, S.I. 2014/1106 added:
On 5 Mar 2014, S.I. 2014/1106* proposed to add:
Lisdexamfetamine
US: CSA Schedule II Section d Subsection 5 Lisdexamfetamine
Effective 1 Jan 1985, S.I. 1984/859 added:
Mecloqualone
CA: CDSA Schedule III Section 4 Mecloqualone
UN: Psychotropics Schedule II Mecloqualone
US: CSA Schedule I Section e Subsection 2 Mecloqualone
Effective 1 Jan 1985, S.I. 1984/859 added:
Methaqualone
CA: CDSA Schedule III Section 3 Methaqualone
UN: Psychotropics Schedule II Methaqualone
US: CSA Schedule I Section e Subsection 3 Methaqualone
⇒
PubChem: 6292; ChemSpider: 6055; Bluelight: Methaqualone; Drugs Forum: Methaqualone; Erowid: Methaqualone; Wikipedia: MethaqualoneEffective 1 May 1998, S.I. 1998/750 added:
Methcathinone
CA: CDSA Schedule III Section 21 2-Methylamino-1-phenyl-1-propanone
UN: Psychotropics Schedule I Methcathinone
US: CSA Schedule I Section f Subsection 5 Methcathinone
⇒
PubChem: 1576; ChemSpider: 1519; Bluelight: methcathinone; Drugs Forum: methcathinone; Erowid: Cathinone and methcathinone; Wikipedia: MethcathinoneEffective 18 Jan 2007, S.I. 2006/3331 revoked:
Methylamphetamine
UK: Currently at MDA Part I Class A Section 1(a) Methylamphetamine
Effective 28 Mar 2011, S.I. 2011/744 revoked:
On 13 Jan 2011, S.I. 2011/744* proposed to revoke:
Effective 16 Apr 2010, S.I. 2010/1207 added:
4-Methylmethcathinone
UK: Currently at MDA Part II Class B Section 1(aa) 2-Amino-1-phenyl-1-propanone
Effective 1 Feb 2002, S.I. 2001/3932 added:
Methylphenidate
CA: CDSA Schedule III Section 2 Methylphenidate
UN: Psychotropics Schedule II Methylphenidate
US: CSA Schedule II Section d Subsection 4 Methylphenidate
⇒
PubChem: 4158; ChemSpider: 4015; Bluelight: Methylphenidate; Drugs Forum: Methylphenidate; Erowid: Methylphenidate; Wikipedia: MethylphenidateEffective 1 Jan 1985, S.I. 1984/859 added:
Methylphenobarbitone
CA: CDSA Schedule IV Section 1 Subsection 18 Methylphenobarbital
UN: Psychotropics Schedule IV Methylphenobarbital
Nicocodine
CA: CDSA Schedule I Section 1 Subsection 24 Nicocodine
UN: Narcotics Part 1 Section 2 Nicocodine and Part 2 Section 1 Nicocodine
US: CSA Schedule I Section c Subsection 19 Nicocodeine
Effective 1 Jul 1973, S.I. 1973/771 added:
Nicodicodine (6-Nicotinoyldihydrocodeine)
UN: Narcotics Part 1 Section 2 Nicodicodine and Part 2 Section 1 Nicodicodine
Norcodeine
CA: CDSA Schedule I Section 1 Subsection 26 Norcodeine
UN: Narcotics Part 1 Section 2 Norcodeine and Part 2 Section 1 Norcodeine
Effective 1 Apr 1986, S.I. 1985/1995 added:
Metazocine
CA: CDSA Schedule I Section 11 Subsection 2 Metazocine
UN: Narcotics Part 1 Section 1 Metazocine and Psychotropics Schedule III Metazocine
US: CSA Schedule II Section c Subsection 14 Metazocine
See also MDA Part I Class A Section 1(a) Metazocine
Phenmetrazine
CA: CDSA Schedule IV Section 6 Phenmetrazine
UN: Psychotropics Schedule II Phenmetrazine
US: CSA Schedule II Section d Subsection 3 Phenmetrazine
Pholcodine
CA: CDSA Schedule I Section 1 Subsection 30 Pholcodine
UN: Narcotics Part 1 Section 2 Pholcodine and Part 2 Section 1 Pholcodine
US: CSA Schedule I Section c Subsection 22 Pholcodine
⇒
PubChem: 5311356; ChemSpider: 4470854; Bluelight: Pholcodine; Drugs Forum: Pholcodine; Wikipedia: PholcodineEffective 26 Feb 2013, S.I. 2013/239 added:
On 8 Jan 2013, S.I. 2013/239* proposed to add:
2-((Dimethylamino)methyl)-1-(3-hydroxyphenyl)cyclohexanol
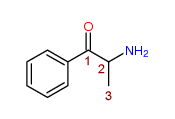
2-Amino-1-phenyl-1-propanone
Cathinone
Diethylpropion
Pyrovalerone
[Bupropion (above) is explicitly not captured by this clause]
4-Methylmethcathinone; Mephedrone, one of many new cathinone analogues this amendment adds to Class B.
Flephedrone, one of many new cathinone analogues this amendment adds to Class B.
Methylone, one of many new cathinone analogues this amendment adds to Class B.
Butylone, one of many new cathinone analogues this amendment adds to Class B.
MDPV, one of many new cathinone analogues this amendment adds to Class B.
Effective 16 Apr 2010, S.I. 2010/1207 added:
1(aa)
Any compound (not being Bupropion, Cathinone, Diethylpropion, Pyrovalerone or a compound for the time being specified in sub-paragraph (a) above) structurally derived from 2-Amino-1-phenyl-1-propanone by modification in any of the following ways, that is to say,
- by substitution in the phenyl ring to any extent with alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents, whether or not further substituted in the phenyl ring by one or more other univalent substituents;
- by substitution at the 3-position with an alkyl substituent;
- by substitution at the nitrogen atom with alkyl or dialkyl groups, or by inclusion of the nitrogen atom in a cyclic structure.
Only a handful of the Cathinone analogues captured by this amendment are shown below. For a more comprehensive enumeration, I highly recommend Synchronium’s elegant depiction, an inspiring model of information design.
CA: CDSA Schedule III Section 19 Cathinone
UN: Psychotropics Schedule I Cathinone
US: CSA Schedule I Section f Subsection 3 Cathinone
See also MDA Part III Class C Section 1(a) Cathinone
CA: CDSA Schedule IV Section 4 Diethylpropion
UN: Psychotropics Schedule IV Amfepramone (Diethylpropion)
US: CSA Schedule IV Section f Subsection 2 Diethylpropion
See also MDA Part III Class C Section 1(a) Diethylpropion
CA: CDSA Schedule IV Section 26 Pyrovalerone
UN: Psychotropics Schedule IV Pyrovalerone
US: CSA Schedule V Section d Subsection 1 Pyrovalerone
See also MDA Part III Class C Section 1(a) Pyrovalerone
EU: Substances Substances 4-Methylmethcathinone
US: CSA Schedule I Section d Subsection 36 4-Methylmethcathinone
⇒
PubChem: 45266826; ChemSpider: 21485694; Bluelight: Mephedrone; Drugs Forum: Mephedrone; Erowid: 4-Methylmethcathinone / Mephedrone; Wikipedia: MephedroneUS: CSA Schedule I Section h Subsection 17 4-Fluoro-N-methylcathinone
⇒
PubChem: 49853406; ChemSpider: 21477355; Drugs Forum: Flephedrone; Erowid: 4-Fluoromethcathinone (4-FMC); Wikipedia: 4-FluoromethcathinoneUS: CSA Schedule I Section d Subsection 47 3,4-Methylenedioxy-N-methylcathinone
US: CSA Schedule I Section h Subsection 14 Butylone
CA: CDSA Schedule I Section 17.1 Methylenedioxypyrovalerone
EU: Substances Substances 3,4-Methylenedioxypyrovalerone
US: CSA Schedule I Section d Subsection 37 3,4-Methylenedioxypyrovalerone
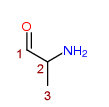
2-Aminopropan-1-one
Naphthylpyrovalerone (Naphyrone), one of many new cathinone analogues this amendment adds to Class B.
Effective 21 Jul 2010, S.I. 2010/1833 added:
1(ab)
Any compound structurally derived from 2-Aminopropan-1-one by substitution at the 1-position with any monocyclic, or fused-polycyclic ring system (not being a phenyl ring or alkylenedioxyphenyl ring system), whether or not the compound is further modified in any of the following ways, that is to say,
- by substitution in the ring system to any extent with alkyl, alkoxy, haloalkyl or halide substituents, whether or not further substituted in the ring system by one or more other univalent substituents;
- by substitution at the 3-position with an alkyl substituent;
- by substitution at the 2-amino nitrogen atom with alkyl or dialkyl groups, or by inclusion of the 2-amino nitrogen atom in a cyclic structure.
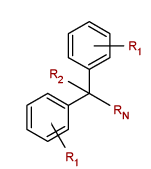
[Generic Diphenylmethyl heterocycle.
R1 = H, alkyl, alkoxy, haloalkyl or halide;
R2 = H, alkyl, hydroxyalkyl or hydroxy;
RN = piperidine, pyrrolidine, azepane, morpholine or pyridine.
The heterocycle nitrogen may have an alkyl, alkenyl, haloalkyl or hydroxyalkyl substituent, though it is unclear if this applies to pyridine where nitorgen is fully engaged by the ring.]
R1 = H, alkyl, alkoxy, haloalkyl or halide;
R2 = H, alkyl, hydroxyalkyl or hydroxy;
RN = piperidine, pyrrolidine, azepane, morpholine or pyridine.
The heterocycle nitrogen may have an alkyl, alkenyl, haloalkyl or hydroxyalkyl substituent, though it is unclear if this applies to pyridine where nitorgen is fully engaged by the ring.]
Effective 13 Jun 2012, S.I. 2012/1390 added:
On 28 Feb 2012, S.I. 2012/1390* proposed to add:
1(ac)
Any compound (not being Pipradrol structurally derived from piperidine, pyrrolidine, azepane, morpholine or pyridine by substitution at a ring carbon atom with a diphenylmethyl group, whether or not the compound is further modified in any of the following ways, that is to say,
- by substitution in any of the phenyl rings to any extent with alkyl, alkoxy, haloalkyl or halide groups;
- by substitution at the methyl carbon atom with an alkyl, hydroxyalkyl or hydroxy group;
- by substitution at the ring nitrogen atom with an alkyl, alkenyl, haloalkyl or hydroxyalkyl group.
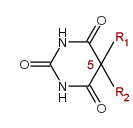
[Barbituric acid with the 5,5-disubstitution pattern. R1 and R2 are typically alkyl, alkenyl, bromoalkenyl, cycloalkenyl or phenyl groups.]
Effective 1 Jan 1985, S.I. 1984/859 added:
1(b)
Any 5,5-disubstituted Barbituric acid
CA: CDSA Schedule IV Section 1 Barbiturates
Effective 23 Dec 2009, S.I. 2009/3209 added:
1(c)
[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone
Effective 23 Dec 2009, S.I. 2009/3209 added:
3-Dimethylheptyl-11-hydroxyhexahydrocannabinol [sic]
The chemical name provided by the legislation seems dodgy. The structure shown is based on an alternative name, HU 243, mentioned in the Advisory Council on the Misuse of Drugs (ACMD) Consideration of the major cannabinoid agonists. This document provides a great overview of the wealth of synthetic cannabinoid recepter agonists. Curiously, parts of the report are redacted, “…on the ground that its publication would not be in the public interest.” Would that be the same “public interest” that led to the sacking of Dr. David Nutt, head of the ACMD?
L.A. King calls this name “technically incorrect.” He is being diplomatic. The correct name, he says, is: 9-(Hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,8,9,10,10a-hexahydro-6H-benzo[c]chromen-1-ol. It’s pretty easy to see how mistakes like that happen.
L.A. King calls this name “technically incorrect.” He is being diplomatic. The correct name, he says, is: 9-(Hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,8,9,10,10a-hexahydro-6H-benzo[c]chromen-1-ol. It’s pretty easy to see how mistakes like that happen.
Effective 23 Dec 2009, S.I. 2009/3209 added:
[9-Hydroxy-6-methyl-3-[5-phenylpentan-2-yl]oxy-5,6,6a,7,8,9,10,10a-octahydrophenanthridin-1-yl] acetate
Effective 23 Dec 2009, S.I. 2009/3209 added:
Effective 23 Dec 2009, S.I. 2009/3209 added:
3-(1-Naphthoyl)indole
3-(2-Naphthoyl)indole
1H-Indol-3-yl-(1-naphthyl)methane
1H-Indol-3-yl-(2-naphthyl)methane
Any compound structurally derived from 3-(1-Naphthoyl)indole, 3-(2-Naphthoyl)indole, 1H-Indol-3-yl-(1-naphthyl)methane or 1H-Indol-3-yl-(2-naphthyl)methane by substitution at the nitrogen atom of the indole ring by alkyl, haloalkyl, alkenyl, cyanoalkyl, hydroxyalkyl, cycloalkylmethyl, cycloalkylethyl, (N-methylpiperidin-2-yl)methyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent.
Effective 26 Feb 2013, S.I. 2013/239 replacement for:
On 8 Jan 2013, S.I. 2013/239* proposed replacement for:
Effective 23 Dec 2009, S.I. 2009/3209 added:
Any compound structurally derived from 3-(1-Naphthoyl)indole or 1H-Indol-3-yl-(1-naphthyl)methane by substitution at the nitrogen atom of the indole ring by alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent.
Special thanks to Nick for alerting me I had overlooked 3-(2-Naphthoyl)indole earlier. I always appreciate it when a reader reports an error or omission. Many eyes…
I looked without success for a representative of 3-(2-Naphthoyl)indole. The ACMD report has this telling rationale:
“In structures containing naphthoyl and adamantoyl groups, positional isomers may also be exploited and, as identification of a particular positional isomer could impose an unnecessary burden on forensic providers, we propose broadening the definitions of controlled compounds of these types to include all possible structures.”
It seems banning an infinite number of substances with totally unknown pharmacology is the price paid to avoid overburdening forensic providers. One hopes by this whim they have not forsaken, say, the next Viagra. Or something even more beneficial.
Please note that there are many, many substances controlled by this paragraph that are not listed below, and the absence of a substance from this tally should not be taken as an indication of legality.
Substances controlled by the 3-(1-Naphthoyl)indole generic definition include: JWH-004, JWH-007, JWH-009, JWH-015, JWH-016, JWH-018, JWH-019, JWH-020, JWH-046, JWH-047, JWH-048, JWH-049, JWH-050, JWH-070, JWH-071, JWH-072, JWH-073, JWH-076, JWH-079, JWH-080, JWH-081, JWH-082, JWH-094, JWH-096, JWH-098, JWH-116, JWH-120, JWH-122, JWH-148, JWH-149, JWH-180, JWH-181, JWH-182, JWH-189, JWH-193, JWH-200, JWH-210, JWH-211, JWH-212, JWH-213, JWH-234, JWH-235, JWH-236, JWH-239, JWH-240, JWH-241, JWH-242, JWH-262, JWH-386, JWH-387, JWH-394, JWH-395, JWH-397, JWH-398, JWH-399, JWH-400, JWH-412, JWH-413, JWH-414, JWH-415, AM-1220 and MAM-2201.
Substances controlled by the 1H-Indol-3-yl-(1-naphthyl)methane generic definition include: JWH-175, JWH-184, JWH-185, JWH-192, JWH-194, JWH-195, JWH-196, JWH-197 and JWH-199.
Listed below are some examples of substances explicitly controlled in other jurisdictions that are members of the group implicitly controlled by this generic definition.
CA: CDSA Schedule II Section 2 Subsection 2 3-(1-Naphthoyl)indole
US: CSA Schedule I Section ii Item ii 3-(1-Naphthoyl)indole
US: CSA Schedule I Section ii Item ii 3-(1-Naphthylmethane)indole
AM-2201
CA: CDSA Schedule II Section 2 Subsection 2 1-(5-Fluoropentyl)-3-(1-naphthoyl)indole
US: CSA Schedule I Section g Subsection 11 1-(5-Fluoropentyl)-3-(1-naphthoyl)indole
JWH-018
CA: CDSA Schedule II Section 2 Subsection 2 1-Pentyl-3-(1-naphthoyl)indole
US: CSA Schedule I Section g Subsection 3 1-Pentyl-3-(1-naphthoyl)indole
JWH-073
CA: CDSA Schedule II Section 2 Subsection 2 1-Butyl-3-(1-naphthoyl)indole
US: CSA Schedule I Section g Subsection 4 1-Butyl-3-(1-naphthoyl)indole
⇒
PubChem: 10471670; ChemSpider: 8647081; Bluelight: JWH-073; Erowid: Spice Product; Wikipedia: JWH-073JWH-081
CA: CDSA Schedule II Section 2 Subsection 2 1-Butyl-3-(4-methoxy-1-naphthoyl)indole
US: CSA Schedule I Section g Subsection 8 1-Pentyl-3-[1-(4-methoxynaphthoyl)]indole
JWH-122
CA: CDSA Schedule II Section 2 Subsection 2 1-Pentyl-3-(4-methyl-1-naphthoyl)indole
US: CSA Schedule I Section g Subsection 9 1-Pentyl-3-(4-methyl-1-naphthoyl)indole
JWH-200
CA: CDSA Schedule II Section 2 Subsection 2 1-(2-Morpholin-4-ylethyl)-3-(1naphthoyl)indole
US: CSA Schedule I Section g Subsection 6 1-[2-(4-Morpholinyl)ethyl]-3-(1-naphthoyl)indole
⇒
PubChem: 10045570; ChemSpider: 8221134; Drugs Forum: JWH-200; Erowid: Spice Product; Wikipedia: JWH-200JWH-398
US: CSA Schedule I Section g Subsection 10 1-Pentyl-3-(4-chloro-1-naphthoyl)indole
Any compound structurally derived from 3-(1-Naphthoyl)pyrrole or 3-(2-Naphthoyl)pyrrole by substitution at the nitrogen atom of the pyrrole ring by alkyl, haloalkyl, alkenyl, cyanoalkyl, hydroxyalkyl, cycloalkylmethyl, cycloalkylethyl, (N-methylpiperidin-2-yl)methyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the pyrrole ring to any extent and whether or not substituted in the naphthyl ring to any extent.
Effective 26 Feb 2013, S.I. 2013/239 replacement for:
On 8 Jan 2013, S.I. 2013/239* proposed replacement for:
Effective 23 Dec 2009, S.I. 2009/3209 added:
Any compound structurally derived from 3-(1-Naphthoyl)pyrrole by substitution at the nitrogen atom of the pyrrole ring by alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the pyrrole ring to any extent and whether or not substituted in the naphthyl ring to any extent.
Please note that there are many, many substances controlled by this paragraph that are not listed below, and the absence of a substance from this tally should not be taken as an indication of legality.
Substances controlled by the 3-(1-Naphthoyl)pyrrole generic definition include: JWH-030, JWH-145, JWH-146, JWH-147, JWH-150, JWH-156, JWH-243, JWH-244, JWH-245, JWH-246, JWH-292, JWH-293, JWH-307, JWH-308, JWH-346, JWH-348, JWH-363, JWH-364, JWH-365, JWH-367, JWH-368, JWH-369, JWH-370, JWH-371, JWH-373 and JWH-392.
CA: CDSA Schedule II Section 2 Subsection 3 3-(1-Naphthoyl)pyrrole
US: CSA Schedule I Section iii Item iii 3-(1-Naphthoyl)pyrrole
Any compound structurally derived from 1-(1-Naphthylmethylene)indene or 1-(2-Naphthylmethylene)indene by substitution at the 3-position of the indene ring by alkyl, haloalkyl, alkenyl, cyanoalkyl, hydroxyalkyl, cycloalkylmethyl, cycloalkylethyl, (N-methylpiperidin-2-yl)methyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indene ring to any extent and whether or not substituted in the naphthyl ring to any extent.
Effective 26 Feb 2013, S.I. 2013/239 replacement for:
On 8 Jan 2013, S.I. 2013/239* proposed replacement for:
Effective 23 Dec 2009, S.I. 2009/3209 added:
Any compound structurally derived from 1-(1-Naphthylmethyl)indene[sic] by substitution at the 3-position of the indene ring by alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indene ring to any extent and whether or not substituted in the naphthyl ring to any extent.
This amendment discretely corrects a significant error in the existing nomenclature which mistakes “methyl” for “methylene.” See structures at left.
I doubt many convictions will be overturned by this blunder, but it seems to me that the substances meant to be captured by this paragraph had, in fact, escaped and remained at large until the amendment came into force.
Please note that there are many, many substances controlled by this paragraph that are not listed below, and the absence of a substance from this tally should not be taken as an indication of legality.
Substances controlled by the 1-(1-Naphthylmethylene)indene generic definition include: JWH-176.
US: CSA Schedule I Section iv Item iv 1-(1-Naphthylmethylene)indene
Any compound structurally derived from 3-Phenylacetylindole by substitution at the nitrogen atom of the indole ring by alkyl, haloalkyl, alkenyl, cyanoalkyl, hydroxyalkyl, cycloalkylmethyl, cycloalkylethyl, (N-methylpiperidin-2-yl)methyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent.
Effective 26 Feb 2013, S.I. 2013/239 replacement for:
On 8 Jan 2013, S.I. 2013/239* proposed replacement for:
Effective 23 Dec 2009, S.I. 2009/3209 added:
Any compound structurally derived from 3-Phenylacetylindole by substitution at the nitrogen atom of the indole ring with alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent.
Please note that there are many, many substances controlled by this paragraph that are not listed below, and the absence of a substance from this tally should not be taken as an indication of legality.
Substances controlled by the 3-Phenylacetylindole generic definition include: JWH-167, JWH-201, JWH-202, JWH-203, JWH-204, JWH-205, JWH-206, JWH-207, JWH-208, JWH-209, JWH-237, JWH-248, JWH-249, JWH-250, JWH-251, JWH-253, JWH-302, JWH-303, JWH-304, JWH-305, JWH-306, JWH-311, JWH-312, JWH-313, JWH-314, JWH-315, JWH-316, and Cannabipiperidiethanone.
CA: CDSA Schedule II Section 2 Subsection 4 3-Phenylacetylindole
US: CSA Schedule I Section v Item v 3-Phenylacetylindole
JWH-250
CA: CDSA Schedule II Section 2 Subsection 4 1-Pentyl-3-(2-methoxyphenylacetyl)indole
US: CSA Schedule I Section g Subsection 7 1-Pentyl-3-(2-methoxyphenylacetyl)indole
Effective 23 Dec 2009, S.I. 2009/3209 added:
Any compound structurally derived from 2-(3-Hydroxycyclohexyl)phenol by substitution at the 5-position of the phenolic ring by alkyl, alkenyl, cycloalkylmethyl, cycloalkylethyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the cyclohexyl ring to any extent.
Please note that there are many, many substances controlled by this paragraph that are not listed below, and the absence of a substance from this tally should not be taken as an indication of legality.
Substances controlled by the 2-(3-Hydroxycyclohexyl)phenol generic definition include: CP-47-497, CP-55,940 and CP-56,667.
Listed below are some examples of substances explicitly controlled in other jurisdictions that are members of the group implicitly controlled by this generic definition.
US: CSA Schedule I Section i Item i 2-(3-Hydroxycyclohexyl)phenol
CP 47,497
CA: CDSA Schedule II Section 2 Subsection 1 5-(1,1-Dimethylheptyl)-2-(3-hydroxycyclohexyl)phenol
US: CSA Schedule I Section g Subsection 1 5-(1,1-Dimethylheptyl)-2-[(1R,3S)-3-hydroxycyclohexyl]-phenol
⇒
PubChem: 15942731; ChemSpider: 10205286; Drugs Forum: CP 47,497; Erowid: Spice Product; Wikipedia: CP 47,497CP 47,497-C8 (Cannabicyclohexanol)
US: CSA Schedule I Section g Subsection 2 5-(1,1-Dimethyloctyl)-2-[(1R,3S)-3-hydroxycyclohexyl]-phenol
Effective 26 Feb 2013, S.I. 2013/239 added:
On 8 Jan 2013, S.I. 2013/239* proposed to add:
Any compound structurally derived from 3-Benzoylindole by substitution at the nitrogen atom of the indole ring by alkyl, haloalkyl, alkenyl, cyanoalkyl, hydroxyalkyl, cycloalkylmethyl, cycloalkylethyl, (N-methylpiperidin-2-yl)methyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent.
Please note that there are many, many substances controlled by this paragraph that are not listed below, and the absence of a substance from this tally should not be taken as an indication of legality.
Substances potentially controlled by the 3-Benzoylindole generic definition include: AM-679, AM-694, AM-2233, RCS-4, RCS-4 (C4), RCS-2 (2-isomer) and WIN 48,098 (Pravadoline)
CA: CDSA Schedule II Section 2 Subsection 5 3-Benzoylindole
US: CSA Schedule I Section v Item v 3-Benzoylindole
AM-694
US: CSA Schedule I Section g Subsection 12 1-(5-Fluoropentyl)-3-(2-iodobenzoyl)indole
Effective 26 Feb 2013, S.I. 2013/239 added:
On 8 Jan 2013, S.I. 2013/239* proposed to add:
Any compound structurally derived from 3-(1-Adamantoyl)indole or 3-(2-Adamantoyl)indole by substitution at the nitrogen atom of the indole ring by alkyl, haloalkyl, alkenyl, cyanoalkyl, hydroxyalkyl, cycloalkylmethyl, cycloalkylethyl, (N-methylpiperidin-2-yl)methyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indole ring to any extent and whether or not substituted in the adamantyl ring to any extent.
Please note that there are many, many substances controlled by this paragraph that are not listed below, and the absence of a substance from this tally should not be taken as an indication of legality.
Substances potentially controlled by the 3-(1-Adamantoyl)indole generic definition include: AB-001 and AM-1248.
Effective 26 Feb 2013, S.I. 2013/239 added:
On 8 Jan 2013, S.I. 2013/239* proposed to add:
Any compound structurally derived from 3-(2,2,3,3-Tetramethylcyclopropylcarbonyl)indole by substitution at the nitrogen atom of the indole ring by alkyl, haloalkyl, alkenyl, cyanoalkyl, hydroxyalkyl, cycloalkylmethyl, cycloalkylethyl, (N-methylpiperidin-2-yl)methyl or 2-(4-morpholinyl)ethyl, whether or not further substituted in the indole ring to any extent.
Please note that there are many, many substances controlled by this paragraph that are not listed below, and the absence of a substance from this tally should not be taken as an indication of legality.
Substances potentially controlled by the 3-(2,2,3,3-Tetramethylcyclopropylcarbonyl)indole generic definition include: UR-144 and XLR-11.
UR-144
CA: CDSA Schedule II Section 2 Subsection 6 (1-Pentyl-1H-indol-3-yl)(2,2,3,3-
tetramethylcyclopropyl)-methanone
US: CSA Schedule I Section h Subsection 1 (1-Pentyl-1H-indol-3-yl)(2,2,3,3-
tetramethylcyclopropyl)methanone
US: [proposed] CSA Schedule I Section g Subsection 16 (1-Pentyl-1H-indol-3-yl)(2,2,3,3-
tetramethylcyclopropyl)methanone
XLR11
CA: CDSA Schedule II Section 2 Subsection 6 [1-(5-Fluoro-pentyl)-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)-methanone
US: CSA Schedule I Section h Subsection 2 [1-(5-Fluoro-pentyl)-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)methanone
US: [proposed] CSA Schedule I Section g Subsection 17 [1-(5-Fluoro-pentyl)-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)methanone
Effective 26 Feb 2013, S.I. 2013/239 added:
On 8 Jan 2013, S.I. 2013/239* proposed to add:
1(d)
1-Phenylcyclohexylamine or any compound (not being ketamine, tiletamine or a compound for the time being specified in paragraph 1(a) of Part 1 of this Schedule) structurally derived from 1-Phenylcyclohexylamine or 2-Amino-2-phenylcyclohexanone by modification in any of the following ways, that is to say, <
ol><
li>by substitution at the nitrogen atom to any extent by alkyl, alkenyl or hydroxyalkyl groups, or replacement of the amino group with a 1-piperidyl, 1-pyrrolidyl or 1-azepyl group, whether or not the nitrogen containing ring is further substituted by one or more alkyl groups;by substitution in the phenyl ring to any extent by amino, alkyl, hydroxy, alkoxy or halide substituents, whether or not further substituted in the phenyl ring to any extent by substitution in the cyclohexyl or cyclohexanone ring by one or more alkyl substituents; by replacement of the phenyl ring with a thienyl ring.
Listed below are some examples of substances explicitly controlled in other jurisdictions that are members of the group implicitly controlled by this generic definition.
Methoxetamine
EU: Substances Substances 2-(3-Methoxyphenyl)-2-(ethylamino)cyclohexanone
UK: Previously at MDA Temporary Class Drug Orders 2012 2-(Ethylamino)-2-(3-methoxyphenyl)cyclohexanone
⇒
PubChem: 52911279; ChemSpider: 24721792; Bluelight: Methoxetamine; Drugs Forum: Methoxetamine; Erowid: Methoxetamine; Wikipedia: Methoxetamine1-[1-(4-Methylphenyl)cyclohexyl]piperidine
CA: CDSA Schedule III Section 23 1-[1-(4-Methylphenyl)cyclohexyl]piperidine
1-Phenyl-N-propylcyclohexanamine
CA: CDSA Schedule III Section 15 1-Phenyl-N-propylcyclohexanamine
TCPy (1-[1-(2-Thienyl)cyclohexyl]pyrrolidine)
US: CSA Schedule I Section d Subsection 35 1-[1-(2-Thienyl)cyclohexyl]pyrrolidine
Effective 10 Jun 2014, S.I. 2014/1106 added:
On 5 Mar 2014, S.I. 2014/1106* proposed to add:
1(e)
Any compound (not being a compound for the time being specified in paragraph 1(ba) of Part 1 of this Schedule) structurally derived from 1-Benzofuran, 2,3-Dihydro-1-benzofuran, 1H-Indole, Indoline, 1H-Indene, or Indane by substitution in the 6-membered ring with a 2-ethylamino [sic] substituent whether or not further substituted in the ring system to any extent with alkyl, alkoxy, halide or haloalkyl substituents and whether or not substituted in the ethylamino side-chain with one or more alkyl substituents.
1-(Benzofuran-5-yl)-propan-2-amine
UK: Previously at MDA Temporary Class Drug Orders 2013 1-(Benzofuran-5-yl)-propan-2-amine
1-(2,3-Dihydro-1-benzofuran-5-yl)-propan-2-amine
UK: Previously at MDA Temporary Class Drug Orders 2013 1-(2,3-Dihydro-1-benzofuran-5-yl)-propan-2-amine
1-(2,3-Dihydro-1-benzofuran-6-yl)-propan-2-amine
UK: Previously at MDA Temporary Class Drug Orders 2013 1-(2,3-Dihydro-1-benzofuran-6-yl)-propan-2-amine
1-(1H-Indol-5-yl)-1-methylethylamine
EU: Substances Substances 5-(2-Aminopropyl)indole
UK: Previously at MDA Temporary Class Drug Orders 2013 1-(1H-Indol-5-yl)-1-methylethylamine
⇒
PubChem: 55253543; ChemSpider: 25991467; Erowid: 5-IT (5-(2-Aminopropyl)indole); Wikipedia: 5-(2-Aminopropyl)indole1-(1H-Indol-6-yl)-1-methylethylamine
UK: Previously at MDA Temporary Class Drug Orders 2013 1-(1H-Indol-6-yl)-1-methylethylamine
2A
Any ester or ether of cannabinol or of a cannabinol derivative or of a substance for the time being specified in paragraph 1(ac), (c) or (d) of this Part of this Schedule.
Effective 26 Feb 2013, S.I. 2013/239 replacement for:
On 8 Jan 2013, S.I. 2013/239* proposed replacement for:
Any ester or ether of cannabinol or of a cannabinol derivative or of a substance for the time being specified in paragraph 1(ac) or (c) of this Part of this Schedule.
Effective 13 Jun 2012, S.I. 2012/1390 replacement for:
On 28 Feb 2012, S.I. 2012/1390* proposed replacement for:
Any ester or ether of cannabinol or of a cannabinol derivative or of a substance for the time being specified in paragraph 1(c) of this Part of this Schedule.
Effective 23 Dec 2009, S.I. 2009/3209 replacement for:
Effective 26 Jan 2009, S.I. 2008/3130 added:
Any ester or ether of cannabinol or of a cannabinol derivative.
3
Any salt of a substance for the time being specified in paragraph 1, 2 or 2A of this Part of this Schedule.
Effective 26 Jan 2009, S.I. 2008/3130 replacement for:
Any salt of a substance for the time being specified in paragraph 1 or 2 of this Part of this Schedule.
11 March 2016 ·  · Isomer Design
· Isomer Design
 · Isomer Design
· Isomer Design